Yeah! Science! For the first time, scientists at Berkeley Lab have synthesized element 116 (livermorium) using a titanium particle beam. Previously, physicists created livermorium atoms using a calcium beam. The new method is a significant step towards creating an entirely new element.
Scientists at the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab), renowned for discovering 16 of the 118 known elements, have taken a significant step towards potentially creating another: element 120.
An international research team led by Berkeley Lab’s Heavy Element Group announced they have successfully synthesized superheavy element 116 using a titanium beam. This breakthrough, presented at the Nuclear Structure 2024 conference, is pivotal for creating element 120. The team published a pre-print version of its detailed findings in Cornell University’s arXiv as the academic journal Physical Review Letters peer-reviews the study for official publication.
“This reaction had never been demonstrated before, and it was essential to prove it was possible before embarking on our attempt to make 120,” said Jacklyn Gates, a nuclear scientist at Berkeley Lab. “Creation of a new element is an extremely rare feat. It’s exciting to be a part of the process and to have a promising path forward.”
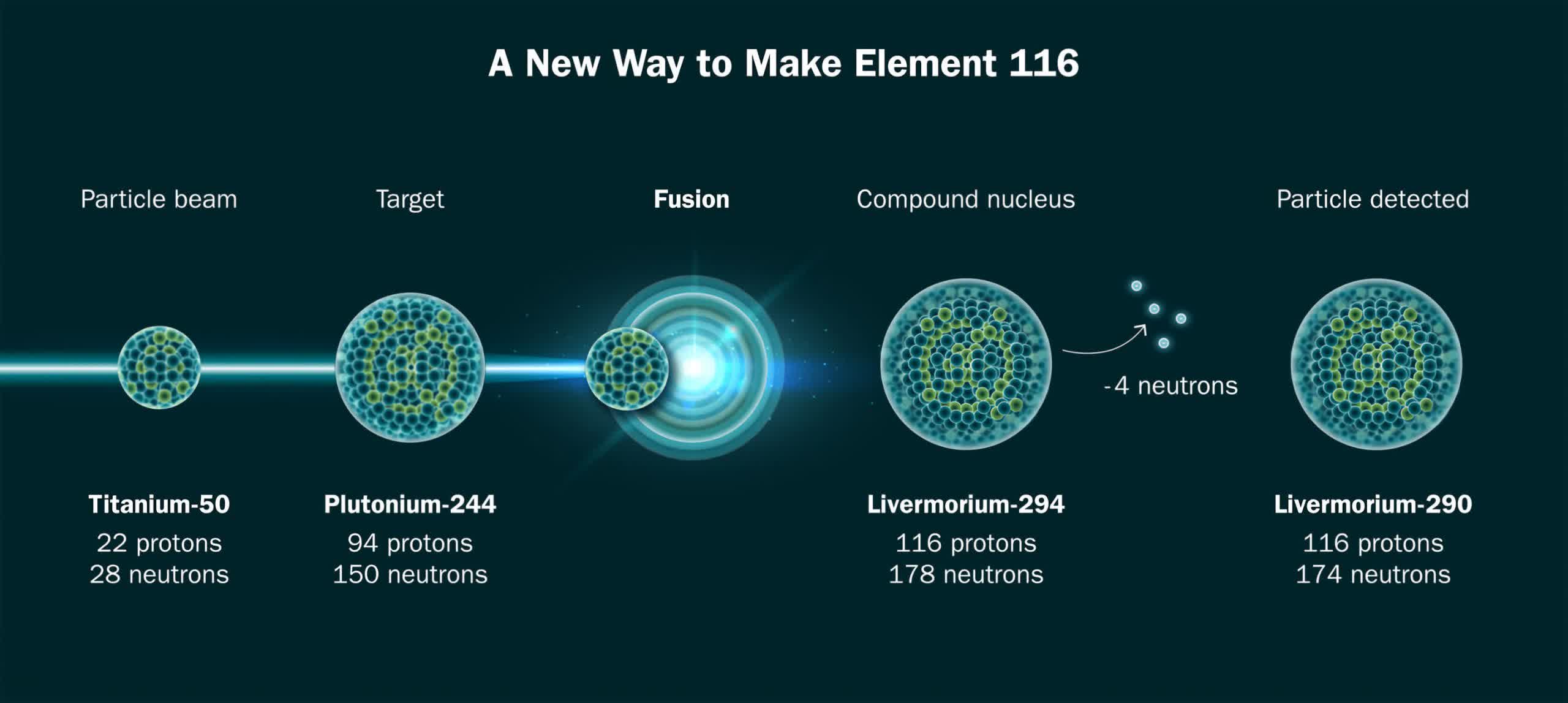
The team produced two atoms of element 116, livermorium, during 22 days of operations at the lab’s heavy-ion accelerator, the 88-inch Cyclotron. The physicists expect the successful synthesis of element 120 to be even rarer, potentially taking 10 times longer than element 116.
“We needed for nature to be kind, and nature was kind,” said Reiner Kruecken, director of Berkeley Lab’s Nuclear Science Division. “It’s not easy, but it seems feasible now.”
If discovered, element 120 would be the heaviest atom created, positioned on the eighth row of the periodic table. It falls on a theorized region of superheavy elements called the “island of stability.” Atoms on the island have unique properties. Unlike the superheavy elements discovered so far, which break apart almost instantaneously, a stable combination of protons and neutrons could create a longer-lasting nucleus, allowing for in-depth study.
The process of creating superheavy elements involves fusing two lighter elements. However, this is incredibly challenging, often requiring trillions of interactions before successful fusion. The heaviest practical target for this experiment is californium-249, with 98 protons. To make element 120, researchers need a beam of titanium-50 atoms, marking a departure from the commonly used calcium-48 beam.
“It was an important first step to try to make something a little bit easier than a new element to see how going from a calcium beam to a titanium beam changes the rate at which we produce these elements,” said Jennifer Pore, a scientist in Berkeley Lab’s Heavy Element Group. “Creating element 116 with titanium validates this method of production works, and we can now plan our hunt for element 120.”
The plan to make superheavy elements using Berkeley Lab’s unique facilities is included in the Nuclear Science Advisory Committee’s 2023 Long-Range Plan for Nuclear Science.
About 6 trillion titanium ions hit the target every second, producing superheavy elements separated by magnets and identified by the Super Heavy RECoil (SHREC) detector. The SHREC apparatus detects several metrics, including energy, location, and time, to allow researchers to identify the heavy element as it decays into lighter particles.
“We’re very confident that we’re seeing element 116 and its daughter particles,” Gates stated, referring to the team’s findings.
Preparations for the attempt to create element 120 continue, with experiments beginning next year. However, the effort could take several years, possibly only producing a few atoms of element 120.
“We want to figure out the limits of the atom, and the limits of the periodic table,” Gates said. “The superheavy elements we know so far don’t live long enough to be useful for practical purposes, but we don’t know what the future holds.”
Image credits: Berkeley Lab