Forward-looking: Approximately five percent of adults listed for a heart transplant in the US die each year while waiting for a suitable donor heart. One of these patients, however, beat the odds by participating in an FDA Early Feasibility Study for an artificial heart created by the medical device company BiVACOR. Implanted with this total artificial heart – a new device that looks nothing like earlier generations of artificial hearts – the patient was able to survive eight days until a transplant. The company hopes its device can be approved for long-term use one day.
Earlier this month, a heart patient received a fully mechanical heart made with maglev technology, similar to that used in high-speed rail lines. The surgery, which was part of an FDA Early Feasibility Study at the Texas Heart Institute, was successful: it kept him alive until he could receive a heart transplant eight days later.
BiVACOR, a clinical-stage medical device company, created the mechanical heart, marking its first-in-human implantation. “It is rewarding to see this result and having the BiVACOR total artificial heart (TAH) perform as expected,” said Dr. Joseph Rogers, President and CEO of the Texas Heart Institute and National Principal Investigator of the research. “The patient continues to do well through their recovery, demonstrating the potential impact of the BiVACOR TAH on the future of heart failure treatment.”
The device represents a significant advancement in artificial heart technology compared to previous designs. The TAH is a titanium-constructed biventricular rotary blood pump with a single magnetically levitated rotor, replacing both ventricles of a failing heart.
Traditional artificial hearts, in contrast to the TAH, mimic the natural beating heart with flexible diaphragms, membranes, and valves. Or put another way: for those eight days the patient’s artificial heart whirled instead of beated.
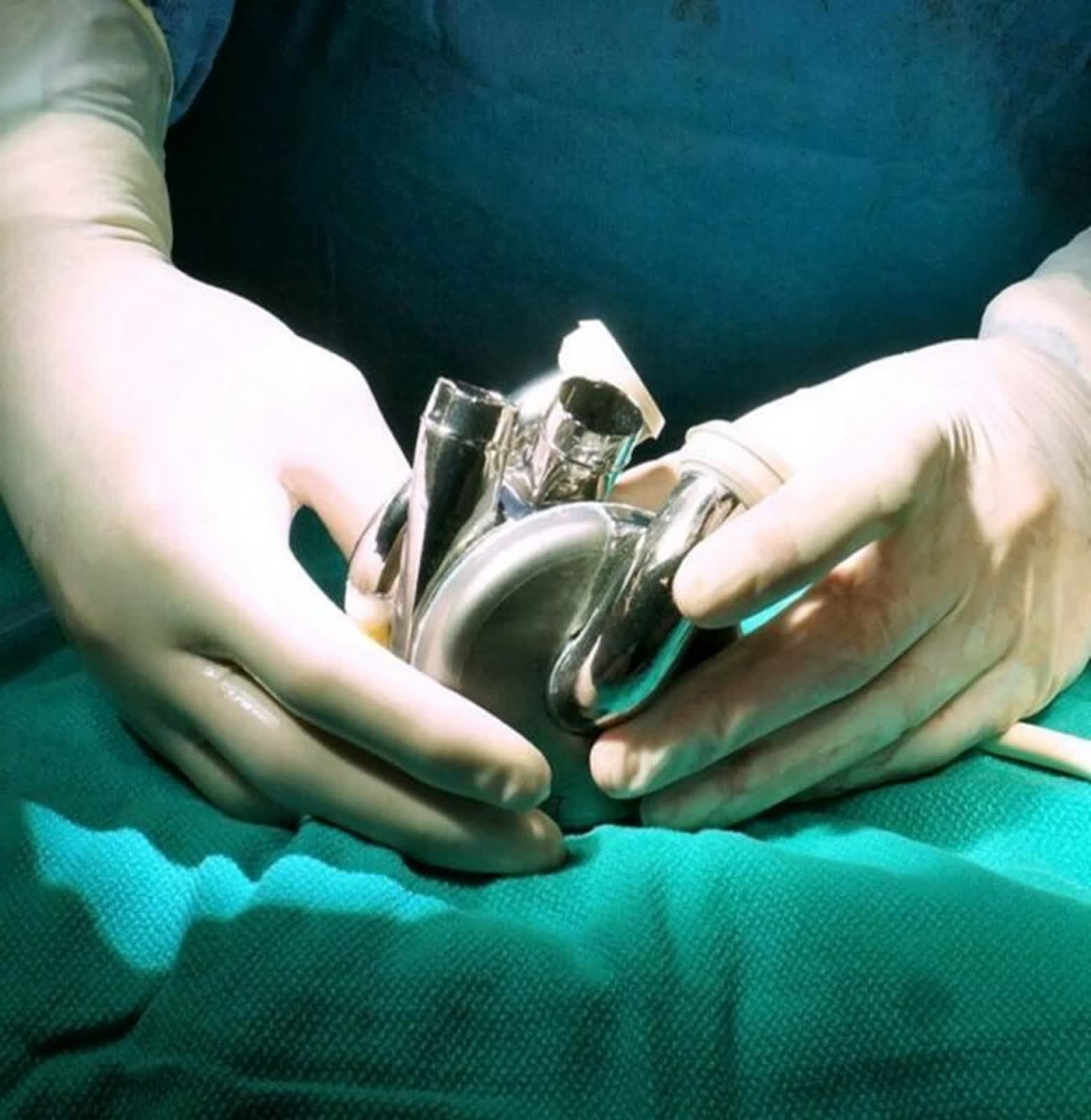
Another advantage the TAH offers over earlier devices is that it is designed to be blood-friendly and has inherently balanced blood flows, capitalizing on the advantages of rotary blood pump technology
The company is planning additional trials, with an eye towards its potential future use as a long-term total heart replacement – something that the TAH’s design supports, at least in theory.
Traditional artificial hearts with flexible components typically last only 12 to 18 months before breaking down due to the repetitive motion, or about 52 million beats per year. The TAH’s design with a rapidly spinning member is expected to pump indefinitely. Also, the device uses much less energy compared to traditional artificial hearts, which is a significant advantage for long-term use.
Currently, the odds are against heart patients. Heart failure affects at least 26 million people worldwide, including 6.2 million adults in the US. Heart transplantations, however, are reserved for those with severe heart failure and are limited to fewer than 6,000 procedures per year globally. The US National Institutes of Health estimates that up to 100,000 patients could immediately benefit from a ventricular assist device (VAD) or TAH.
“We anticipate the BiVACOR TAH may eventually save numerous lives and improve the quality of life for patients who otherwise have no alternative therapy available,” said Dr. Alexis Shafii, Surgical Director of Heart Transplantation at Baylor St. Luke’s Medical Center and Associate Professor of Surgery, Cardiothoracic Transplant & Circulatory Support at Baylor College of Medicine.
With this first implantation successfully completed, four additional patients are set to be enrolled in the study.

